What is a ”blackbody”? The natural and common-sensical answer to the question asked here would be ‘’a body looking perfectly black’’. But it is not the color of the body that has earned it the name. In fact, the name is not-at-all ‘’self-explanatory’’. It is a scientific term used to denote a body that absorbs all the radiation falling on it, irrespective of frequency and the angle of incidence, so that it looks black when it is not emitting. This is because if a body absorbs all the light without either reflecting any of it or emitting any radiation of its own, it has to look ‘’black’’ as no light from the body is reaching our eyes.
A blackbody looks really ‘’black’’ only when it is not emitting in the visible spectrum. So, if a so-called ‘’blackbody’’ has to stick to its name, it cannot emit any visible radiation. But is that possible is the question. As far as choice is concerned, a blackbody has no choice, whether to emit or not, but to follow the laws of nature. The laws of nature seem to state that any body has to emit some radiation and the spectrum of radiation depends on the temperature of the body. Hence, whether a blackbody apparently looks black depends on the temperature of the body. Let us dig deeper into the issue to understand the science behind it.
If a body is non-isolated and is in thermal contact with its surrounding, there is a regular transfer of energy both ways from one to the other. This transition phase, when there is a net directional transfer of energy from one side to the other, is a non-equilibrium phase when the temperature of the body does not remain constant. It is either rising or decreasing depending on the direction of energy transfer. During this phase, the body has to emit some radiation irrespective of the net direction of energy transfer, if the same has to take place through a radiative process. But emission during such a phase is not what we consider when we define a ‘’blackbody’’. When we say that a blackbody is one which absorbs all the radiation falling on it, we are saying that it absorbs all of them while being at thermal equilibrium with the surrounding. The surrounding is important as the radiation absorbed by the body is actually emitted by the surrounding. The proper definition, therefore, would be that ”a blackbody is one which absorbs all the radiation falling on it and emits according to a universal law, when maintained at thermal equilibrium with its surrounding”.
The visible radiation is only a small part of the total electromagnetic spectrum which consists of mostly invisible radiation like x-rays, ultraviolet, infrared, microwaves, and so on. A body will not look invisible even if it emits invisible radiation. If the radiation emitted by a blackbody lies mostly in the invisible part of the spectrum, the body will look black to the naked eyes, as black color is really the absence of visible colors. A body is invisible only when it is transparent to the radiation incident on it. If a body is transparent to the visible radiation, then it is invisible in the visible spectrum or with the naked eyes. But the same body may be visible if it is looked at another wavelength, say, infrared or ultraviolet where it is emitting. Moreover, we do not always get to see what a body emits. Rather, we are mostly seeing what a body reflects. But in the case of a blackbody, there is no reflection to be seen. The only way we can see a blackbody is through their emissions. This makes a blackbody special. If the radiation spectrum of the blackbody peaks at the red color, then the blackbody will appear red-hot. Whereas it looks green if the radiation spectrum peaks near the green wavelength. So, it is the temperature that decides how a blackbody appears to the naked eye.
An ideal blackbody fully conforming to the scientific definition is not found in practice. We can find bodies that only approximate this ideal behavior. Carbon black or soot is an example of an approximate blackbody, so is our sun. The experimentally observed spectrum of a blackbody looks as shown below.
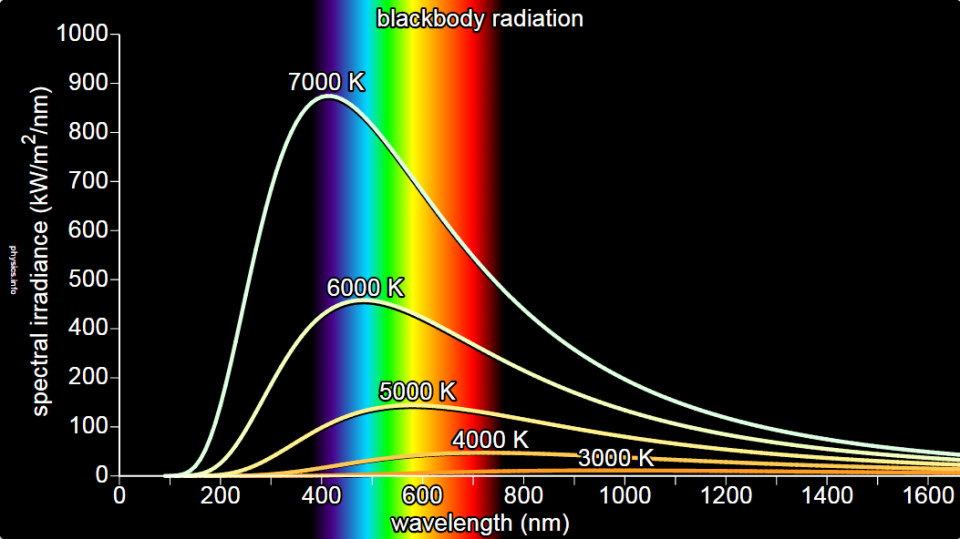
But this experimentally observed spectrum gave a hard time to the physicists of the 19th century. They could not arrive at a successful explanation using the theory of classical electromagnetism. The classical theory predicted a blackbody spectrum which looks as shown in the figure below.

This spectrum matched the longer wavelength part of the spectrum but was in sharp disagreement with the smaller wavelength part of the spectrum as seen in the figure above. Moreover, it made an unrealistic prediction that an infinite amount of total energy should be radiated by a blackbody. This can be inferred from the infinite area under the curve predicted by the classical theory. The point of departure of the classical prediction from the observed blackbody spectrum lies near the ultraviolet region of the spectrum. This phenomenon was, therefore, called the ultraviolet catastrophe. The disagreement between experiment and theory made physicists realize that classical theory is not the complete theory. Something more fundamental has to be responsible for the behavior of radiation and matter as exposed by their mutual interaction in the form of blackbody radiation.
Two other phenomena called the Photoelectric effect and the Compton Scattering also presents such disagreement with the classical prediction. But let us stick to the blackbody radiation for the present. Max Planck was a devoted classical physicist specializing in the field of thermodynamics. He never fully bought the idea, originally conceived by Ludwig Boltzmann, of using statistics of microscopic particles composing a macroscopic system to predict its macroscopic properties. But in 1900, he had to cook up an unusual assumption, which was not allowed by the classical theory, besides accepting the statistical ideas to finally derive the radiation formula by theoretical means. His assumption was not based on scientific reasoning but guided by speculation and anticipation as to what would yield the desired formula. Later, the assumptions made by Planck on the discreteness of the packets of energy that could be transferred from radiation to matter or vice-versa proved epochal and gave way to the development of the modern theory of Quantum Mechanics.
The derivation has been refined and modified by many physicists after the initial work of Planck. His derivation has been shown to be consisting of ad-hoc assumptions which are no longer accepted in the original form after the development of the modern theory. But the ground laid by Planck deserves a high degree of scientific reverence. During the initial years of the 20th century, physicists heavily researched and exchanged their views on both the mathematical and philosophical perspectives on the subject, which led to the present form of Quantum Mechanics. The present form is largely accepted in the scientific community, except the disagreements on the larger philosophy of the theory. We shall explore the mathematical details of the derivation of the blackbody radiation formula, as accepted today, in a future article. Till then, we should surely give a thought to the probability of how even assumptions, initially made to explain an empirical observation, guided by no scientific logic or reason, can become the basis for a ‘’quantum’’ leap in our fundamental understanding of nature.

